Alkylphosphocholines and Quaternary Ammonium Compounds against Acanthamoeba Keratitis
Ronnie Mooney*, Roderick Williams
Institute of Biomedical and Environmental Health Research, University of the West of Scotland, School of Health and Life Sciences, High Street, Paisley, PA1 2BE, Scotland, UK
Abstract
Acanthamoeba keratitis (AK) is a sight threatening infection caused by the free-living amoeba Acanthamoeba. This infection is largely associated with contact lens wear and the recent increase in AK incidences highlights the ineffectiveness of existing curative and preventative treatments. Current curative and protective treatments being active in part, only against the infective trophozoites and often inducing their conversion to the protective cysts is a major issue, particularly when the latter are the main cause of disease resurgences and relapses. These point to the need for the discovery of new drugs for curative and preventive treatments. Two structurally similar chemical classes, alkylphosphocholines (APCs) and quaternary ammonium compounds (QACs) that address these issues will be discussed in this review.
Introduction
Acanthamoeba are free-living single celled eukaryotes, existing in two forms; an infective trophozoite and a dormant cyst. They are ubiquitously found in both natural and man-made environments and are of clinical relevance due to their opportunistic parasitic activities in humans1. There are several Acanthamoeba associated infections in humans, but this review will focus on the sight threatening disease of the eye Acanthamoeba keratitis (AK)2 and the use of two promising compounds as curative and preventive treatments that will validate its incorporation into contact lens solutions. Curative treatment is the use of systemic oral drugs and topical eye drops while preventive treatment employs medical devices e.g. contact lens solutions to sterilise contaminated lenses and prevent contact lens to eye transmission.
AK is largely associated with improper contact lens hygiene and the limited activity of contact lens solutions against Acanthamoeba. The attachment of Acanthamoeba to contact lens allows transmission to, and infection of the cornea arising from the feeding activities of trophozoites in the eye3. Incidence rates of AK have been increasing globally since the start of the 21st century. On average, the annual number of AK cases within the Moorfields eye hospital, England increased from 12.8 to 48.6 between 2000-2008 and 2009-20164, and 16 to 49 in the Netherlands between 2009 and 20155. Similar reports are documented in Australia and the USA with rising incidences, the Sydney Eye Hospital reported the annual average cases of AK to be 2.6 and 3.6 before and after 20076, while cases in Iowa, USA increased in average annual cases from 2.9 to 6.5 between 2002-2009 and 2010-2017 respectively7. The reasons for this spatio-temporal increase are not readily apparent but improved diagnostic techniques and ineffective contact lens cleansing solutions are thought to be key factors4.
Existing curative and preventive treatments for AK mainly target trophozoites but become inactive if they induce their conversion to cysts via the process of encystation8. The cellulose cell walls of cysts protect the parasite against drug activity9,10 and the reduction of drug concentrations in the eye to sub-lethal levels initiates the reversion of cysts to the trophozoite stage, ultimately leading to disease resurgence10.
The inability of current topical and oral drugs and many widely used contact lens solutions to produce death at low concentrations against cysts, the requirement of a prolonged treatment regime to ensure complete clearance from the eye and the ability to induce encystation are factors that have made AK treatment and prevention difficult11. In some instances, frequent application of drugs topically for up to a year is required, causing corneal damage and very often patients require surgical intervention e.g. corneal transplant to repair the damaged cornea10,12. Preventive protocols with commonly used contact lens solutions such as one-step hydrogen peroxide solutions are similarly ineffective with even compliant users at risk13,14.
Two structurally similar compounds named alkylphosphocholines (APCs) and quaternary ammonium compounds (QACs) have received attention for their activity against several protozoan pathogens, including Acanthamoeba15-20. These compounds are active against cysts and synergistic with other antimicrobials including Acanthamoeba spp15-21. Work by Walochnik and colleagues on their anti-amoebic properties for combating Acanthamoeba keratitis concluded that APCs are promising drug candidates that might prove useful against AK21. This minireview provides an update and the state of the art of this chemical class and an analogue called QACs, extensively used as preventative strategy against AK.
Alkylphosphocholines against Acanthamoeba Keratitis
Structure of alkylphosphocholines
APCs are zwitterionic molecules comprised of a head made from a trimethylamine moiety containing a positively charged nitrogen atom and joined by a two alkyl-carbon linker to a negatively charged phosphoryl group. The tail, made of varied number of alkyl-carbons, is attached to the phosphoryl group (Figure 1).
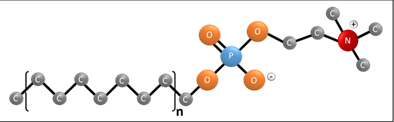
Figure 1: General structure of an alkylphosphocholine. The molecule is comprised of a negatively charged phosphoryl group and positively charged nitrogen joined by a linker made from two alkyl-carbons which forms a neutrally charged head group. The tail is comprised of varied length of alkyl-carbon chain with different degree of unsaturation and can be with sulphur and oxygen substitutions for the hydrogen joined to the phosphoryl moiety. “n” indicates the variable alkyl-carbon tail.
Activity of oral miltefosine against Acanthamoeba keratitis
The APC, miltefosine was first developed as an anti-cancer drug but was subsequently shown to have anti-fungal and anti-protozoal properties20,22-27. Systemic use of miltefosine was licensed in the USA as a treatment of AK in 2016 and when incorporated into treatment regimens at 50mg/ml, given three times daily (TD) for up to two months has proved to be an effective oral systemic treatment against Acanthamoeba mainly in combination15,28 (Table 1). The use of miltefosine solely as an oral administration is yet to be tested, but in combination with topical applications e.g. chlorhexidine (0.06%) and propamidine (0.1%) has demonstrated success15. Hirabayashi et al.15 reported the use of miltefosine in the treatment of a patient suffering with progressively worsening AK. For this 17yr old female patient, earlier treatments with topical polyhexamethylene biguanide (PHMB, 0.02%), chlorhexidine (0.02%), moxifloxacin (0.5%), cyclopentolate (1%) and oral administration of both 500mg valacyclovir and 200mg voriconazole twice daily (BD) were unsuccessful. The addition of miltefosine (50mg, TD, orally) in particularly with topical chlorhexidine (0.06%) and propamidine isethionate (0.1%) for five weeks reduced the infection and accompanying symptoms, pain improved conjunctival injection and corneal opacity were reduced and improved respectively15. No resurgence was observed one-year post treatment15 (Table 1). Similar results have been described by Dewan et al28 , again a 44yr old female who did not respond to treatment with topical chlorhexidine (0.02%), PHMB (0.02%,), gatifloxacin (0.3%) and voriconazole (0.5mg/ml) and oral voriconazole (200mg, BD) was responsive to oral miltefosine (50mg, TD) after 11 months of treatment28 (Table 1). Resurgence was not evident thirty-month post treatment28. The efficacy of miltefosine is reproducible and has been replicated in part by Naranjo et al.29. Oral miltefosine (50mg, TD) was effective and reduced parasite load in the eye of the patient after three weeks, with no re-infection noted29 (Table 1). However, in four out of six cases, inflammatory responses produced either by the eye immunity towards the killed Acanthamoeba or the immunomodulatory effect of miltefosine produced deterioration in the eye which was corrected by penetrating keratoplasty29. Studies of the combination of this compound with anti-inflammatory medication is required to determine if managing the immune response could improve treatment, although it has been suggested that miltefosine might be used as an immune modulating anti-inflammatory compound so an extensive investigation of this inflammatory effect would be required30.
Table 1: Oral miltefosine against Acanthamoeba keratitis
|
Participant specifics |
Treatment regimen prior to miltefosine usage |
Treatment regimen with miltefosine |
Remarks |
Ref. |
|
17-year old female |
T1: Several Months - Antivirals and topical corticosteroids administered for several months. T2: 2 weeks – Topical; PHMB (0.02%; q1h)/Chlorhexidine (0.02%; QD), Moxifloxacin (0.5%; TD), cyclopentoate (1%; OD), Oral; valacyclovir (500mg; OD) T3: 6 weeks; - Oral; Valacyclovir discontinued and replaced voriconazole (200mg; BD) |
T4: 1 week – Topical; PHMB (0.02%; q1h)/Chlorhexidine (0.02%; QD), Moxifloxacin (0.5%; QD), cyclopentoate (1%; TD), Oral; miltefosine (50mg; TD) T5: Undisclosed time – Topical; PHMB (0.02%; QD)/Chlorhexidine (0.02%), Oral; miltefosine (50mg; TD), doxycline (50mg; BD), OD Vitamin C (1000mg; OD) T6: 5 weeks - Topical; PHMB was discontinued while treatment in T5 updated with Chlorhexidine (0.06%; q1h) and propamidine isethionate (0.1%; q2h) T7: Undisclosed time – Topical; Chlorhexidine (0.06%; q2h), Oral; BD Miltefosine (50mg; BD) T8: 2 months – Topical; Chlorhexidine (0.06%; QD)/propamidine isetionate (0.1%, QD), Oral; Miltefosine (50mg; OD) T9: 1 year - Miltefosine discontinued but treatment described in T8 continued for 1 year |
- Cultures were negative for Acanthamoeba after T9. - Pain, conjunctival injection and cornea opacity decreased significantly after five weeks treatment with miltefosine (50mg; TD oral), chlorhexidine (0.06%; q1h topical), propamidine isethionate (0.1%; q2h topical), doxycline (50mg; BD oral) and Vitamin C (1000mg; OD oral) (See T6) - No recurrence was observed one year post treatment with miltefosine (see T9) |
15 |
|
44-year old female |
T1: 4 weeks (inconsistently) - Topical; prednisolone acetate (1%; q1h), dexamethasone sodium phosphate ointment (0.1%; OD), homatropine (2%; BD) T2: 2 weeks - Topical; tobramycin (0.3%; OD), dexamethasone ophthalmic ointment (0.1%; OD), gatifloxacin (0.3%; QD) T3: Undisclosed time - Topical; chlorhexidine (0.02%; q1h)/PHMB (0.02%;q1h), gatifloxacin (0.3%; QD) T4: 3 weeks - Topical; prednisolone acetate (1%; QD), voriconazole (0.5mg.ml; q1h) added, Oral; voriconazole (200mg; BD) |
T5: 3 months - Topical; Voriconazole (0.5mg) discontinued, chlorhexidine (0.02%;q1h)/PHMB (0.02%;q1h), gatifloxacin (0.3%; QD), prednisolone acetate (1%; QD) Oral; Miltefosine (50mg; TD), Voriconazole (200mg; BD) T6: 3 months - Topical; prednisolone acetate (1%; q2h), chlorhexidine (0.02%; q2h), PHMB (0.02%; q2h) Oral; Miltefosine (50mg; BD), Voriconazole (200mg; BD) |
- Cultures were native for Acanthamoeba after T6 - Standard treatments for AK (T1 to T4) were unsuccessful, but the addition of miltefosine in T5 to T6 cleared the infection - No disease resurgence observed 30 months post treatment (T6) |
28 |
|
29-year old female |
T1: 2 days - Topical; ganciclovir gel (n/a), erythromycin ointment (n/a), Oral; valacyclovir (n/a) T2: 5 days - Topical; empiric fortified vancomycin (n/a), tobramycin eye drops (n/a) T3: 2 weeks - Topical; fortified vancomycin discontinued, chlorhexidine (n/a), PHMB (n/a), polymyxin/trimethoprim (n/a) added |
T4: 15 days - Topical; chlorhexidine (n/a), PHMB (n/a), polymyxin/trimethoprim (n/a) Oral; miltefosine (50mg; TD) T5: 13 days - Topical; PHMB (n/a), prednisolone acetate (1%) propamidine (n/a) Oral; see T4 |
- Cultures were negative for Acanthamoeba afrer T5 - Patients symptoms worsened after 6 days treatment with miltefosine, predicted to be immune related (T4) - Cultures returned negative for Acanthamoeba, only one cyst was identified in histopathological analysis - No disease recurrence noted 12 months post treatment (T5) |
29 |
|
53-year old female |
T1: Undisclosed time - Topical; ganciclovir ophthalmic gel (0.15%), prednisolone acetate (1%), Oral; valacyclovir (n/a) T2: Several months - Topical; chlorhexidine (n/a), propamidine (n/a) |
T3: 15 days - Topical; chlorhexidine (n/a), Oral; miltefosine (50mg; TD) T4: 20 days - Topical; chlorhexidine (n/a), PHMB (n/a), Oral; see T3 |
- Cultures were negative for Acanthamoeba afrer T4 - Symptoms worsened after 15 days treatment with miltefosine, presumed immune related (T3) - no disease recurrence noted 5 months post treatment (T4) |
29 |
|
24-year old female |
T1: 3 weeks - Topical; ganciclovir ophthalmic gel (0.15%) T2: 2 weeks - Topical; ganciclovir ophthalmic gel (0.15%) prednisolone (n/a), Oral; valacyclovir (n/a) T3: 20 days - Topical; PHMB (n/a; q1h) added, Oral; see T2 |
T4: 28 days - Topical; ganciclovir ophthalmic gel (0.15%), prednisolone, PHMB (n/a; q4h), Oral; Miltefosine (50mg; BD), valacyclovir (n/a) |
- Cultures were negative for Acanthamoeba afrer T4 - Symptoms worsened after 12 days treatment with miltefosine, presumed immune related (T4) - no disease recurrence noted 9 months post treatment (T4) |
29 |
|
25-year old female |
T1: Several weeks - Topical; ganciclovir ophthalmic gel (n/a) ofloxacin drops (n/a), Oral; famcyclovir (n/a) T2: 40 days - Topical; propamidine isethionate (n/a), PHMB (n/a), polymyxin/trimethoprim (n/a) Oral; valacyclovir (n/a) |
T3: 28 days - Topical; propamidine isethionate (n/a), PHMB (n/a), polymyxin/trimethoprim (n/a) Oral; Miltefosine (50mg; TD), fluconazole (n/a) |
- Cultures were negative for Acanthamoeba afrer T3 - Symptoms improved after 8 days treatment with miltefosine (T3) - no disease recurrence noted 9 months post treatment (T3) |
29 |
|
16-year old female |
T1: Several weeks - Topical; prednisolone acetate (n/a), Oral; acyclovir (n/a) T2: 1 month - Topical; PHMB (n/a), chlorhexidine (n/a), Oral; see T1 T3: 2 months - Topical; chlorhexidine discontinued, propamidine isethionate (n/a) added Oral; see T1 |
T4: 28 days - Topical; PHMB (n/a), propamidine isetionate (n/a), Oral; Miltefosine (50mg; BD), acyclovir (n/a) |
- Cultures were negative for Acanthamoeba afrer T4 - Symptoms improved after 20 days treatment with miltefosine (T4) - no disease recurrence noted 9 months post treatment (T4) |
29 |
|
55-year old female |
T1: Undisclosed time - Topical; erythromycin (n/a), besifloxacin (n/a), valacyclovir (n/a), and difluprednate (n/a) T2: Undisclosed time – Topical; moxifloxacin (n/a), bacitracin (n/a), polymyxin B (n/a) T3: 6 weeks – Topical; moxifloxacin (n/a), bacitracin (n/a), polymyxin B (n/a) discontinued, PHMB (n/a), propamidine isethionate (n/a), cyclopentolate eye drops (n/a) added |
T4: 28 days - Topical; PHMB (n/a), propamidine isethionate (n/a), cyclopentolate eye drops (n/a) Oral; Miltefosine (50mg; TD) T5: 1 month - Topical; PHMB (n/a), steroids (n/a) Oral; Miltefosine (50mg) discontinued
|
- Cultures were negative for Acanthamoeba afrer T5 - Symptoms worsened after 21 days treatment with miltefosine, presumed immune related (T4) - no disease recurrence noted 8 weeks post treatment (T5) |
29 |
|
59-year old male |
T1: 1 month - Topical; ofloxacin (n/a) T2: 2 weeks - Topical; PHMB (0.02%; q1h), Oral; voriconazole (400mg; BD), reduced (200mg and eventually discontinued within this time T3: 1 week - Topical; PHMB (0.02%; q2h), dexamethasone (0.1%) T4: Undisclosed time - Topical; PHMB (0.02%; q1h) T5: 2 weeks - Topical; PHMB (0.06%; q1h), hexamidine (0.1%; q1h), levofloxacin (0.5%; q1h) T6: 4 weeks - Topical; hexamidine (0.1%), levofloxacin (0.5%) discontinued, PHMB (0.06%; QD), prednisolone (0.5; QD) added T7: 2 months - Topical; QD PHMB (0.02%), QD dexamethasone (0.1%) T8: 2 months - Topical; q1h PHMB (0.02%), q1h chlorhexidine (0.02%), q1h hexamidine (0.1%), q1h cefuroxime (5%), q1h gentamicin (1.5%) T9: Undisclosed time - Topical; q1h chlorhexidine (0.2%), q1h imidazole (1%), QD cefuroxime (5%), QD gentamicin (1.5%), QD predforte (1%) Oral; BD posaconazole (300mg), BD tacrolimus (1mg), Intravenous; methylprednisolone (1g), Intracameral; voriconazole (0.5μg/ml) |
T9: 2 weeks - Topical; chlorhexidine (0.2%; q1h), imidazole (1%; q1h), cefuroxime (5%; QD), gentamicin (1.5%; QD), predforte (1%; QD) Oral; tacrolimus (1mg) discontinued, Miltefosine (50mg; TD), posaconazole (300mg; BD),
|
- Cultures were negative for Acanthamoeba afrer T9 - Miltefosine administered prior to surgery to prevent the spread of Acanthamoeba to the CNS (T9) - Amoeba were not cleared but no spread was documented (T9) - Amoeba present in the vitreous but further spread to the retina, choroid and optic nerve was prevented, not clear if this is a result of miltefosine treatment however (T9)
|
32 |
Key: Tn – Treatment intervention sequence where n is number of intervention, OD - once daily, BD - twice daily, TD - three times daily, QD - four times daily, qxh - taken every x hours, where x is the duration.
Miltefosine is yet to be licensed for AK treatment in UK, but clinical trials to prevent trophozoites migration from the eye to the central nervous system, resulting in granulomatous amoebic encephalitis (GAE), an infection with a mortality rate of 90%31, has proven successful32. Preliminary results based on histopathological analysis showed that while migration was halted, the infection was not cleared from the eye. However, treatment with miltefosine was only administered for two-weeks prior to surgery32. These results show that the treatment of AK is complicated and marred with issues of toxicity and dosage regime (Table 1) but confirms that miltefosine is a bona fide treatment for AK in combination with the standard topical treatments particularly when they are ineffective.
Activity of topical miltefosine against Acanthamoeba keratitis
The success of oral miltefosine suggests that it can be absorbed easily by the gut, is able to survive the first pass effect in the liver and the bioavailability in the eye is of sufficient concentration to cause Acanthamoeba death. This suggests that lower doses would be sufficient for topical use. Work done by Polat et al.33 demonstrated that the topical application of miltefosine alone to the Acanthamoeba-infected eyes of Syrian hamsters for 28 days at 160μM cleared AK infections by 85%33 (Table 2). In contrast, the current recommended topical treatment combinations of 0.1% propamidine isetionate and 0.02% PHMB was less effective in the eyes of Syrian hamsters than miltefosine alone33. The same group reported further benefits for the use of miltefosine as a combined topical treatment against Acanthamoeba keratitis in rats34. Miltefosine (160μM) combined with PHMB (0.02%) or chlorhexidine (0.02%) but not propamidine isethionate (0.1%) showed synergistic activity34 (Table 2). This study illustrated that existing topical treatments with PHMB can be improved by the addition of topical miltefosine. We thus propose that miltefosine should be integrated with current treatment regimens for improved prognosis for patients.
Table 2: Topical miltefosine against Acanthamoeba keratitis
|
Experimental details |
Preparation |
Experimental treatments |
Key Findings |
Ref. |
|
AK eye model from 40 male Syrian hamsters divided into 3 groups |
Miltefosine prepared at 2mM dissolved in 5% ethanol |
Group 1 - Miltefosine (160μM) Group 2 - Propamidine isethionate (0.1%) and PHMB (0.02%) combination Infected Control group - Ethanol (0.05%) in PBS Each regime was used to treat Acanthamoeba-infected eyes for 28 days |
Group 1 – 85% of eyes were normal with cultures of excised post-treatment tissues negative for Acanthamoeba Group 2 – 65% of eyes were normal with 5% cultures of excised post infection tissues positive for Acanthamoeba. Infected Control group – 5% of eyes were normal with 6% of culture of excised tissues positive for Acanthamoeba. Conclusion; Topical miltefosine was more effective in treating AK than propamidine isethionate and PHMB combinations often used in treating AK. |
33 |
|
AK eye model from 63 male Wistar rats divided into 7 groups. |
2mM of Miltefosine prepared at 2mM in 5% ethanol |
Group 1 - Miltefosine (160μM)
Group 2 – chlorhexidine gluconate (0.02%)
Group 3 – PHMB (0.02%)
Group 4 – propamidine isethionate (0.1%)
Group 5 – Miltefosine (160μM) and chlorhexidine gluconate (0.02%) combination
Group 6 – Miltefosine (160μM) and PHMB (0.02%) combination
Group 7 – Miltefosine (160μM) and propamidine isethionate (0.1%) combination
Infected Control group - Ethanol in PBS (0.05%)
Each regime was used to treat Acanthamoeba-infected eyes for 28 days |
Group 1 – 14.2% and 50% of eyes were normal or almost normal respectively with 28.5% cultures of excised tissues positive for Acanthamoeba.
Group 2 – 7.1% and 50% of eyes were normal or almost normal respectively with 28.5% of cultures of excised tissues positive for Acanthamoeba.
Group 3 – 7.1% and 42.8% of eyes were normal or almost normal respectively with 21.4% cultures of excised tissues positive for Acanthamoeba.
Group 4 – 7.1% and 35.5% of eyes were normal or almost normal with 28.5% cultures of excised tissue positive for Acanthamoeba.
Group 5 –14.2% and 57.1% of eyes were normal or almost normal respectively with 21.4% cultures of excised tissues positive for Acanthamoeba.
Group 6 – 28.4% and 64.2% of eyes were normal or almost normal respectively with 14.2% cultures of excised tissue positive for Acanthamoeba.
Group 7 – 7.1% and 35.5% of eyes were normal or almost normal respectively with 21.4% of cultures of excised tissues positive for Acanthamoeba.
Infected Control group - 0% and 21.4% of eyes were normal or almost normal respectively with 71.4% cultures of excised tissues positive for Acanthamoeba.
Conclusion; Topical treatments of miltefosine combined with PHMB or chlorhexidine were synergistic; while combinations with propamidine isethionate were additive. PHMB-miltefosine combination is an effective treatment for AK. |
34 |
In vitro activity of APCs against Acanthamoeba
There are several in vitro studies describing the efficacy of APC analogues against Acanthamoeba with most showing miltefosine (hexadecylphosphocholine) to have optimal activity against Acanthamoeba trophozoites (Table 3). In a structure activity relationship study undertaken by Mooney and colleagues, a series of APCs with different alkyl-carbon chain length ranging from 8-18 carbons, demonstrated that miltefosine was cytotoxic at 46μM and cytostatic below this dosage against trophozoites35. Another study showed that 39mM to 78mM of miltefosine was toxic to cysts36. Similar results reported elsewhere for miltefosine16,17,35,37 have shown that the activity of miltefosine against Acanthamoeba is influenced by the life form16,17,35,36, the Acanthamoeba species16,37, the strain36, and the duration of the drug in contact with the protist36. Nevertheless, they have shown that approximately 3000-fold lower concentrations of miltefosine was required for in vitro treatment than for topical and oral application13,32,33 (Table 3). This makes a good case for their incorporation into contact lens solutions as a preventive strategy. Studies show that human tissues such as the eye, organotypic skin models and mammalian breast cancer cells are refractory to miltefosine concentrations 160µM, 50μM and 150mM respectively19,33,34,38,39 which suggest that it could be safe to use as a preventive strategy and eye toxicity would not be an issue.
Table 3: In vitro APCs and QACs against Acanthamoeba
|
Class |
Compound name |
Experimental conditions |
Key Findings |
Ref. |
|
APCs |
||||
|
APC: |
Hexadecyl-PC (Miltefosine), Octadeyl-PC, Eicosanyl-PC, (Z)-12-heneicosenyl-PC, (Z)-13-docosenyl-PC, (Z)-10-docosenyl-PC, (Z,Z)-6, 12-eicosadienyl-PC, (Z,Z)-6,15-tetracosadienyl-PC |
A. castellanii, A. polyphaga, A. lenticulata trophozoites and cysts treated with miltefosine at concentration ranging from 5-160μM for 72 hours. |
Findings: Miltefosine was most effective against A. castellanii and A. polyphaga trophozoites (MIC, 40μM) and A. lenticulata trophozoites 80μM. These concentrations killed 60%-80% of cysts of all strains. Conclusions: Miltefosine had species-specific activity against Acanthamoeba at low micromolar concentrations in vitro. |
16 |
|
APC: |
Hexadecyl-PC (Miltefosine) |
Trophozoites of A. castellanii, A. polyphaga (2 strains), Unknown Acanthamoeba sp. treated with miltefosine at concentrations ranging from 10-80μM for 1 week |
Findings: Miltefosine killed Acanthamoeba spp. at concentrations above 40μM with recovery within 1-2 weeks below this concentration. Conclusion: Miltefosine had concentration-dependent cytostatic and cytotoxic activity. |
17 |
|
APC: |
Hexadecyl-PC (Miltefosine) |
Cysts of three environmental isolates within T3, T4 and T5 genotypes were treated with miltefosine at concentrations ranging from 2.42-77.44mM for 1 week |
Findings: minimal cysticidal concentration for cysts of the T4 and T5 genotypes were killed after 1 day at 38.72mM while for T3 cysts, it was 77.44mM. At 7 days it decreased 9.68mM, 4.84mM and 4.84mM for the T3, T4 and T5 genotypes respectively. Conclusion: The species- and life form-specific activity of miltefosine against Acanthamoeba was time dependent. Activity on environmental isolates was significantly higher than lab-derived strains. |
36 |
|
APC: |
Hexadecyl-PC (Miltefosine), octadecyl-PC, elaidyl-PC, erucyl-PC, edelfosine |
Trophozoites of A. castellanii, A. polyphaga incubated with miltefosine at concentrations ranging from 7.8-1000μM for 96 hours. |
Findings: The order of activity against Acanthamoeba spp. was Hexadecyl-PC > octadecyl-PC albeit with A. castellanii more susceptible than A. polyphaga. MIC for hexadecyl-PC was 62.5μM and 125μM for A. castellanii and A. polyphaga respectively. Conclusion: Species-specific activity of miltefosine against Acanthamoeba was highlighted, vital to inform a preventative strategy. |
37 |
|
QACs |
||||
|
nbQAC: |
Polyquad-1 |
Cysts of A. castellanii, A. polyphaga, A. hatchetti treated with 0.001% Polyquad-1 formulated in the contact lens solutions Alcon Opti-Clean II, Alcon Opti-Free Express, Alcon Opti-Free Replenish for 24 hours |
Findings: Viable cysts were observed for all 3 Acanthamoeba spp. after 24 hours incubation in all Polyquad-1 -contact lens solution formulations Conclusion: Polyquad-1 concentrations in contact lens solutions are inactive against Acanthamoeba cysts |
11 |
|
bQAC: |
Benzalkonium chloride (BAC) |
Trophozoites and cysts of A. castellanii, A. polyphaga treated with BAC at concentrations ranging from 2.7-134μM (0.005-0.02%) for 48 hours in the BAC containing solutions Levofloxacin (Oftaquix; 0.005% BAC), trifluorothymidine ( 0.02% BAC) |
Findings: BAC monotherapy was effective in killing trophozoites at concentrations of 0.02μM and 0.005μM and cysts at concentrations of 0.04μM and 0.02μM for A.castellanii and A.polyphaga respectively. The Levofloxacin (Oftaquix) ophthalmic solution with 0.005% BAC has a minimal amoebicidal concentration of 156μg/ml and 312μg/ml and cysticidal concentration of 625μg/ml and 625μg/ml for A.castellanii and A. polyphaga respectively. For TMT containing 0.02% BAC these same respective concentrations were 625μg/ml and 1250μg/ml for trophozoites and 5000μg/ml and 2500μg/ml for cysts. Conclusion: BAC is highly potent to Acanthamoeba trophozoites and cysts and addition to contact lens solutions significantly increases efficacy. |
42 |
|
bQAC:
nbQAC: |
Benzalkonium chloride (BAC)
Polyquad-1 |
Trophozoites and cysts of A. castellanii (2 strains), A. polyphaga (2 strains), A. mauritaniensis (6 strains) at concentrations ranging from 2.7-134μM (0.005-0.02%) for up to 8 hours in the QAC containing solutions, Duracare (0.004% BAC), Optifree (0.001% Polyquad-1), Optisoak (0.05% Polyquad-1), Oxysept 1 Step (0.004% BAC), Transoak (0.01% BAC), Transol Wetting Solution (0.004% BAC) |
Findings: 0.01% BAC in Transoak was most effective and killed cysts from all species and strains within four hours. Lower BAC concentrations present in Duracare (0.004%) and Transol (0.004%) required more than 8 hours to produce similar activity against cysts. Optifree and Optisoak containing Polyquad-1 had no cysticidal activity for all Acanthamoeba species and strain. Conclusion: Benzylated QACs as a medical device demonstrated better activity against Acanthamoeba trophozoites and cysts than their non-benzylated counterpart. |
43 |
|
APC and QAC |
||||
|
nbQAC:
APC: |
Dodecyl-TMAB, Tetradecyl-TMAB, Hexadecyl-TMAB, Octadecyl-TMAB
Dodecyl-PC, Tetradecyl-PC, Hexadecyl-PC (Miltefosine) |
Trophozoites and cysts of A. castellanii at concentrations ranging from 0.5-400μM for 96 hours. |
Findings: Octadecyl-TMAB and miltefosine (hexadecyl-PC) were most effective QAC and APCs against Acanthamoeba trophozoites respectively, with the nbQACs, Octadecyl-TMAB cytotoxic at low concentrations and demonstrated cysticidal activity after 24 hours. Dodecyl-TMAB and miltefosine was synergistic.
Conclusion: nbQACs more effective than APCs and demonstrated higher cysticidal activity but synergistic when combined. |
35 |
|
nbQAC:
bQAC:
APC: |
Cetyl-TMAB
Cetylpyridinium bromide,Benzethonium chloride (BAC) Hexadecyl-PC (Miltefosine), Erucylphosphohomocholine, Perifosine, N-benzyl-N,N-dimethyl-N-hexadecyl-AB |
Trophozoites of A. lugdunensis, A. quina at concentrations ranging from 0.98-500μM for 48 hours |
Findings: The QACs cetyl-TMAB, cetylpyridinium bromide and N-benzyl-N,N-dimethyl-N-hexadecylammonium bromide were most effective against both Acanthamoeba spp with comparable MICs, 15.6μM. Conclusion: QACs are more effective against Acanthamoeba trophozoites than APCs. |
45 |
Key: bQAC – benzylated QAC, nbQAC – non-benzylated QAC, -TMAB – trimethyl ammonium bromide, -PC - Phosphocholine
Quaternary Ammonium Compounds Against Acanthamoeba
Structure and classification of quaternary ammonium compounds
QACs lack the negatively charged phosphoryl group of APCs resulting in a net positive charge. They are cationic molecules containing a positively charged central nitrogen atom attached to four substituents. Attachments to aliphatic and aromatic functional groups have produced two main types of QACs. In this review, they will be referred to as benzylated and non-benzylated if the compound contains an aromatic benzyl ring (e.g. benzalkonium chloride and benzethonium chloride) or lacks one (e.g. tetraethylammonium bromide and dodecyltrimethylammonium bromide) attached to the nitrogen atom respectively (Figure 2).
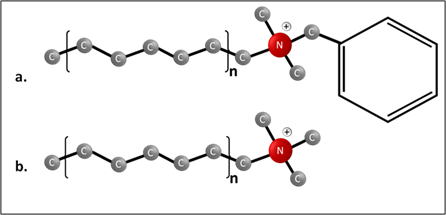
Figure 2: General structures of benzylated QACs. The molecules contain a positively charged nitrogen group attached to (a) two methyl groups and a benzyl ring (benzylated) or (b) three methyl groups (non-benzylated). “n” indicates the variable alkyl-carbon tail.
Activity of quaternary ammonium compounds against Acanthamoeba
Broad antimicrobial properties against virus, bacteria and protozoans have been reported in 1,866 studies since 201540-42. The activity of both QACs against Acanthamoeba has also been reported recently35,43-45. Benzylated QACs such as benzalkonium chloride (BAC) have widespread clinical applications, commonly used in ophthalmic solutions as topical and preventive strategies, and can cause death at concentrations ranging from 2.69μM to 20.97M and 20.97μM to 41.93μM against trophozoites and cysts respectively46, far lower than that in ophthalmic solutions being 107.52μM43. Lower concentrations of benzylated QACs formulated in medical devices with longer incubation times in cytotoxicity assays have increased efficacy31,43. To date, the activity of benzylated QACs against different Acanthamoeba species, strains and their respective life forms have been reported8,41-43. The unusually structured non-benzylated-QAC, Polyquaternium-1, widely used in ophthalmic solutions, has no cysticidal activity against Acanthamoeba11,47. However, other non-benzylated compounds have reported alkyl carbon length dependent efficacy against trophozoites and cysts35. The non-benzylated QAC, dodecyltrimethylammonium bromide (DTAB) with 12 alkyl-carbon atoms was non-toxic against Acanthamoeba trophozoites or cysts at concentrations up to 486μM35. Interestingly, their benzylated counterpart was active at 1.6mM35,48. The 18-carbon analogue, octadecyltrimethylammonium bromide (OTAB) was the most toxic (IC50; trophozoites; 17.6μM and cyst; 38.2μM)35. This structural variation is linked to the ability of the compounds to produce death by micelle formation35. In vitro structure-activity relationship analysis have revealed that the net charge is another major determinant of the activity of this compound against trophozoites; cationic>zwitterionic>anionic35,49. Cationic molecules can rapidly reverse the net negative charge of the plasma membrane to produce shock due to the opposing charge of the molecule50. It has been shown that toxicity to human cells can be an issue, but currently available data only investigates higher concentrations than those recorded as toxic against Acanthamoeba. For example, octadecyltrimethylammonium bromide causes severe eye irritation at concentrations above 510mM, while ~1200-fold (42µM) lower doses are required for activity against Acanthamoeba. Nevertheless, studies are required to validate QACs as a preventative strategy35,51. Should corneal toxicity be an issue, neutralising agents such as β-cyclodextrin could provide a second step during contact lens cleansing52. Two-step methods have been effectively used for hydrogen peroxide based contact lens solutions13.
Conclusion
The impact of APCs in AK treatments, even amongst the ‘difficult to treat’ immuno-suppressed patients is promising15,28,32. Despite the clinically observed effect, little is known about its clinical pharmacodynamics, mainly because good quantitative markers of parasite load and treatment response are not available for AK. Disease-specific pharmacokinetics for miltefosine alone53 and in combination54 are available for other anti-parasitic infections e.g. Leishmaniasis but are yet to be available for AK. Further clinical research is required for these compounds use against AK. The studies highlighted in this review suggest that miltefosine may reduce current prolonged treatment regime and prevent cyst-induced disease resurgence, common with current curative and preventive treatments. Similarly, pharmacodynamics and pharmacokinetic for QACs are scarce and are perhaps hampered by their extensive use as preservatives. The strong efficacy of QACs against Acanthamoeba is a framework for development as a treatment or preventative for AK, if corneal toxicity issues are addressed.
References
- Khan NA. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol Rev. 2006; 30(4): 564–95. doi: 10.1111/j.1574-6976.2006.00023.x.
- Jones DB. Acanthamoeba -The ultimate opportunist? Am J Ophthalmol. 1986; 102(4): 527–30. doi: 10.1016/0002-9394(86)90085-1.
- Lorenzo-Morales J, Martín-Navarro CM, Lopez-Arencibia A, et al. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013; 29(4): 181–7. doi: 10.1016/j.pt.2013.01.006.
- Carnt N, Hoffman JJ, Verma S, et al. Acanthamoeba keratitis: Confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol. 2018; 102(12): 1621–8. doi: 10.1136/bjophthalmol-2018-312544.
- Randag AC, van Rooij J, van Goor AT, et al. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS One. 2019; 14(9): e0222092. doi: 10.1371/journal.pone.0222092.
- Hollhumer R, Keay L, Watson SL. Acanthamoeba keratitis in Australia: demographics, associated factors, presentation and outcomes: a 15-year case review. Eye. 2020; 34(4): 725–32. doi: 10.1038/s41433-019-0589-6.
- Scruggs BA, Quist TS, Salinas JL, et al. Acanthamoeba keratitis cases — Iowa, 2002–2017. Morb Mortal Wkly Rep. 2019; 68(19): 448–9. doi: 10.15585/mmwr.mm6819a6.
- Campbell SJ, Ingram PR, Roberts CW, et al. Induced encystment improves resistance to preservation and storage of Acanthamoeba castellanii. Parasitology. 2008; 135(12): 1401–5. doi: 10.1017/S0031182008005003.
- Roberts CW, Henriquez FL. Drug target identification, validation, characterisation and exploitation for treatment of Acanthamoeba (species) infections. Exp Parasitol. 2010. doi: 10.1016/j.exppara.2009.11.016.
- Mazur T, Hadas E, Iwanicka I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med Parasitol. 1995; 46(2): 106–8. PMID: 8525280.
- Johnston SP, Sriram R, Qvarnstrom Y, et al. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J Clin Microbiol. 2009; 47(7): 2040–5. doi: 10.1128/JCM.00575-09.
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite. 2015; 22: 10. doi: 10.1051/parasite/2015010.
- Hiti K, Walochnik J, Faschinger C, et al. One- and two-step hydrogen peroxide contact lens disinfection solutions against Acanthamoeba: How effective are they? Eye. 2005; 19(12): 1301–5. doi: 10.1038/sj.eye.6701752.
- Fears AC, Metzinger RC, Killeen SZ, et al. Comparative in vitro effectiveness of a novel contact lens multipurpose solution on Acanthamoeba castellanii. J Ophthalmic Inflamm Infect. 2018; 8(1). doi: 10.1186/s12348-018-0161-8.
- Hirabayashi KE, Lin CC, Ta CN. Oral miltefosine for refractory Acanthamoeba keratitis. Am J Ophthalmol Case Reports. 2019; 16. doi: 10.1016/j.ajoc.2019.100555.
- Walochnik J, Duchene M, Seifert K, et al. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother. 2002; 46(3): 695–701. doi: 10.1128/AAC.46.3.695-701.2002.
- Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and Voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp, and Naegleria fowleri. J Eukaryot Microbiol. 2006. doi: 10.1111/j.1550-7408.2005.00082.x.
- Blaha C, Duchene M, Aspock H, et al. In vitro activity of hexadecylphosphocholine (miltefosine) against metronidazole-resistant and -susceptible strains of Trichomonas vaginalis. J Antimicrob Chemother. 2006; 57(2): 273–8. doi: 10.1093/jac/dki417.
- Walochnik J, Obwaller A, Gruber F, et al. Anti-Acanthamoeba efficacy and toxicity of miltefosine in an organotypic skin equivalent. J Antimicrob Chemother. 2009. doi: 10.1093/jac/dkp215.
- Croft SL, Neal RA, Pendergast W, et al. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem Pharmacol. 1987; 36(16): 2633–6. doi: 10.1016/0006-2952(87)90543-0.
- Walochnik J, Duchene M, Eibl H, et al. [Treatment of Acanthamoeba keratitis: possibilities, problems, and new approaches]. Wien Klin Wochenschr. 2003; 115 Suppl: 10–107. PMID: 15508774.
- van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008; 14(21): 2061–74. doi: 10.2174/138161208785294636.
- Timko L, Fischer-Fodor E, Garajova M, et al. Synthesis of structural analogues of hexadecylphosphocholine and their antineoplastic, antimicrobial and amoebicidal activity. Eur J Med Chem. 2015; 93: 263–73. doi: 10.1016/j.ejmech.2015.02.014.
- Llull D, Rivas L, Garcia E. In vitro bactericidal activity of the antiprotozoal drug miltefosine against Streptococcus pneumoniae and other pathogenic streptococci. Antimicrob Agents Chemother. 2007; 51(5): 1844–8. doi: 10.1128/AAC.01428-06.
- Santa-Rita RM, Santos Barbosa H, Meirelles MDNSL, et al. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 2000; 75(2): 219–28. doi: 10.1016/S0001-706X(00)00052-8.
- Tahir M, Bashir U, Hafeez J, et al. Safety and efficacy of miltefosine in cutaneous leishmaniasis: An open label, non-comparative study from Balochistan. Pakistan J Med Sci. 2019; 35(2): 495–9. doi: 10.12669/pjms.35.2.54.
- Souza W de, Godinho J, Barrias E, et al. Effects of Phospholipid Analogues on Trypanosomatids. Mol Biol Kinetoplastid Parasites. 2018; doi: 10.21775/9781910190715.13.
- Dewan N, Ming W, Holland SP, et al. Oral Miltefosine as Adjunctive Treatment for Recalcitrant Acanthamoeba Keratitis. Cornea. 2019; 38(7): 914–7. doi: 10.1097/ICO.0000000000001968.
- Naranjo A, Martinez JD, Miller D, et al. Systemic Miltefosine as an Adjunct Treatment of Progressive Acanthamoeba Keratitis. Ocul Immunol Inflamm. 2020; 1–9. doi: 10.1080/09273948.2020.1758156.
- Verhaar AP, Wildenberg ME, Peppelenbosch MP, et al. Repurposing miltefosine for the treatment of immune-mediated disease? J Pharmacol Exp Ther. 2014; 350(2): 189–95. doi: 10.1124/jpet.113.212654.
- Krol-Turminska K, Olender A. Human infections caused by free-living amoebae. Ann Agric Environ Med. 2017; 24(2): 254–60. doi: 10.5604/12321966.1233568.
- Tavassoli S, Buckle M, Tole D, et al. The use of miltefosine in the management of refractory Acanthamoeba keratitis. Contact Lens Anterior Eye. 2018; 41(4): 400–2. doi: 10.1016/j.clae.2018.03.007.
- Polat ZA, Obwaller A, Vural A, et al. Efficacy of miltefosine for topical treatment of Acanthamoeba keratitis in Syrian hamsters. Parasitol Res. 2012. doi: 10.1007/s00436-011-2515-0.
- Polat ZA, Walochnik J, Obwaller A, et al. Miltefosine and polyhexamethylene biguanide: A new drug combination for the treatment of Acanthamoeba keratitis. Clin Exp Ophthalmol. 2014. doi: 10.1111/ceo.12120.
- Mooney R, Masala M, Martial T, et al. Alkyl-carbon chain length of two distinct compounds and derivatives are key determinants of their anti-Acanthamoeba activities. Sci Rep. 2020; 10(1). doi: 10.1038/s41598-020-62934-8.
- Chao M, Thongseesuksai T, Boonmars T, et al. Investigation of the in vitro cysticidal activity of miltefosine against Acanthamoeba spp. J Parasit Dis. 2020; 44(2): 491–5. doi: 10.1007/s12639-020-01204-w.
- McBride J, Mullen AB, Carter KC, et al. Differential cytotoxicity of phospholipid analogues to pathogenic Acanthamoeba species and mammalian cells. J Antimicrob Chemother. 2007. doi: 10.1093/jac/dkm245.
- Smorenburg CH, Seynaeve C, Bontenbal M, et al. Phase II study of miltefosine 6% solution as topical treatment of skin metastases in breast cancer patients. Anticancer Drugs. 2000; 11(10): 825–8. doi: 10.1097/00001813-200011000-00006.
- Unger C, Peukert M, Sindermann H, et al. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Cancer Treat Rev. 1990; 17(2–3): 243–6. doi: 10.1016/0305-7372(90)90054-J.
- Ito M, Alam MS, Suzuki M, et al. Virucidal activity of a quaternary ammonium compound associated with calcium hydroxide on avian influenza virus, newcastle disease virus and infectious bursal disease virus. J Vet Med Sci. 2018; 80(4): 574–7. doi: 10.1292/jvms.18-0006.
- Alam MS, Takahashi S, Ito M, et al. Bactericidal efficacy of a quaternary ammonium compound with food additive grade calcium hydroxide toward salmonella infantis and escherichia coli on abiotic carriers. J Vet Med Sci. 2018; 80(10): 1482–9. doi: 10.1292/jvms.18-0390.
- Duque-Benitez SM, Ríos-Vasquez LA, Ocampo-Cardona R, et al. Synthesis of Novel Quaternary Ammonium Salts and Their in Vitro Antileishmanial Activity and U-937 Cell Cytotoxicity. Molecules. 2016; 21(4). doi: 10.3390/molecules21040381.
- Cornellas A, Perez L, Comelles F, et al. Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J Colloid Interface Sci. 2011; 355(1): 164–71. doi: 10.1016/j.jcis.2010.11.063.
- Lukac M, Pisarcik M, Lacko I, et al. Surface-active properties of nitrogen heterocyclic and dialkylamino derivates of hexadecylphosphocholine and cetyltrimethylammonium bromide. J Colloid Interface Sci. 2010; 347(2): 233–40. doi: 10.1016/j.jcis.2010.03.041.
- Lukac M, Mrva M, Garajová M, et al. Synthesis, self-aggregation and biological properties of alkylphosphocholine and alkylphosphohomocholine derivatives of cetyltrimethylammonium bromide, cetylpyridinium bromide, benzalkonium bromide (C16) and benzethonium chloride. Eur J Med Chem. 2013; 66: 46–55. doi: 10.1016/j.ejmech.2013.05.033.
- Heaselgrave W, Hamad A, Coles S, et al. In vitro evaluation of the inhibitory effect of topical ophthalmic agents on acanthamoeba viability. Transl Vis Sci Technol. 2019; 8(5). doi: 10.1167/tvst.8.5.17.
- Niszl IA, Markus MB. Anti-Acanthamoeba activity of contact lens solutions. Br J Ophthalmol. 1998; 82(9): 1033–8. doi: 10.1136/bjo.82.9.1033.
- Roy C, Metzinger R, Reimers R. Compositions and methods for multipurpose disinfection and sterilization solutions. US 2019/0224361 A1, 2019.
- Mooney R, Henriquez F, Williams R. Composition. 1911694.6, 2019.
- Vieira DB, Carmona-Ribeiro AM. Cationic lipids and surfactants as antifungal agents: Mode of action. J Antimicrob Chemother. 2006. doi: 10.1093/jac/dkl312.
- ECHA. Trimethyloctadecylammonium bromide - Registration Dossier - ECHA 2016. https://echa.europa.eu/registration-dossier/-/registered-dossier/19273/7/4/3 (accessed July 13, 2020).
- Abdelkader H, Fathalla Z, Moharram H, et al. Cyclodextrin enhances corneal tolerability and reduces ocular toxicity caused by diclofenac. Oxid Med Cell Longev. 2018. doi: 10.1155/2018/5260976.
- Dorlo TPC, Van Thiel PPAM, Huitema ADR, et al. Pharmacokinetics of miltefosine in old world cutaneous leishmaniasis patients. Antimicrob Agents Chemother. 2008; 52(8): 2855–60. doi: 10.1128/AAC.00014-08.
- Omollo R, Alexander N, Edwards T, et al. Safety and Efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: Study protocol for a randomized controlled trial. Trials. 2011; 12. doi: 10.1186/1745-6215-12-166.
